Molecular and genetic dissection of pathogenetic mechanisms in pulmonary inflammation and fibrosis
 Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive and usually lethal lung disorder of unknown etiology, and no proven therapies. Current research indicates that the mechanisms driving IPF reflect abnormal, deregulated wound healing within the lung, involving increased activity and possibly exaggerated responses by a spectrum of pro-inflammatory and pro-fibrogenic factors. To further dissect pathogenetic mechanisms of pulmonary fibrosis, we are utilizing the animal model of bleomycin-induced pulmonary inflammation and fibrosis, precision cut lung slices (PCLS), and 3D ECM fibroblast cell based systems to address the possible involvement of various mediators through genetic modifications and pharmacologic interventions, coupled with respiratory and metabolic functions, expression profiling, as well as translational research.
Idiopathic pulmonary fibrosis (IPF) is a chronic, progressive and usually lethal lung disorder of unknown etiology, and no proven therapies. Current research indicates that the mechanisms driving IPF reflect abnormal, deregulated wound healing within the lung, involving increased activity and possibly exaggerated responses by a spectrum of pro-inflammatory and pro-fibrogenic factors. To further dissect pathogenetic mechanisms of pulmonary fibrosis, we are utilizing the animal model of bleomycin-induced pulmonary inflammation and fibrosis, precision cut lung slices (PCLS), and 3D ECM fibroblast cell based systems to address the possible involvement of various mediators through genetic modifications and pharmacologic interventions, coupled with respiratory and metabolic functions, expression profiling, as well as translational research.
The lab has recently edited a special research topic on “Pulmonary Fibrosis” published at Frontiers in Medicine, available here.
The lab is eligible to receive fellowships from Marie Sklodowska-Curie (MSCA) via the European Respiratory Society (ERS) and the RESPIRE4 project.
Indicative selected publications
- Tzouvelekis A, Harokopos V, Paparountas T, Oikonomou N, Chatziioannou A, Vilaras G, Tsiambas E, Karameris A, Bouros D and Aidinis V., 2007, "Comparative expression profiling in pulmonary fibrosis suggests a role of hypoxia-inducible factor-1alpha in disease pathogenesis", Am J Respir Crit Care Med, 176(11) :1108-19 [Pubmed]
- Oikonomou N, Thanassopoulou A, Tzouvelekis A, Harokopos V, Paparountas T, Nikitopoulou I, Witke W, Karameris A, Kotanidou A, Bouros D, and Aidinis V., 2009, "Gelsolin expression is necessary for the development of modelled pulmonary inflammation and fibrosis.", Thorax. , 64(6): 467-75 [Pubmed].
- Mouratis MA, Aidinis V. 2011, "Modeling pulmonary fibrosis with bleomycin.", Curr Opin Pulm Med., 2011 Sep; 17(5):355-61. [PubMed].
- Oikonomou N, Mouratis MA, Tzouvelekis A, Kaffe E, Valavanis C, Vilaras G, Karameris A, Prestwich GD, Bouros D, Aidinis V. 2012, "Pulmonary Autotaxin Expression Contributes to the Pathogenesis of Pulmonary Fibrosis.", Am J Respir Cell Mol Biol, 2012 Jun 28 [PubMed].
- Ninou I, Magkrioti C, Aidinis V. 2018, "Autotaxin in Pathophysiology and Pulmonary Fibrosis", Front Med, 2018 Jun 13; 5:180 [PubMed].
- Zannikou M, Barbayianni I, Fanidis D, Grigorakaki T, Vlachopoulou E, Konstantopoulos D, Fousteri M, Nikitopoulou I, Kotanidou A, Kaffe E, Aidinis V. MAP3K8 Regulates Cox-2-Mediated Prostaglandin E 2 Production in the Lung and Suppresses Pulmonary Inflammation and Fibrosis. J Immunol. 2021 Feb 1;206(3):607-620. [PubMed]
- Galaris A, Fanidis D, Tsitoura E, Kanellopoulou P, Barbayianni I, Ntatsoulis K, Touloumi K, Gramenoudi S, Karampitsakos T, Tzouvelekis A, Antoniou K, Aidinis V. 2023, "Increased lipocalin-2 expression in pulmonary inflammation and fibrosis.", Front Med., 2023 Sep 7; 10:1195501. [PubMed]
- Barbayianni I, Kanellopoulou P, Fanidis D, Nastos D, Ntouskou ED, Galaris A, Harokopos V, Hatzis P, Tsitoura E, Homer R, Kaminski N, Antoniou KM, Crestani B, Tzouvelekis A, Aidinis V, . 2023, "SRC and TKS5 mediated podosome formation in fibroblasts promotes extracellular matrix invasion and pulmonary fibrosis", Nat Commun., 2023 Sep 21; 14(1):5882. [PubMed]
The role of ATX and LPA signaling in embryonic development, pathophysiology and cancer
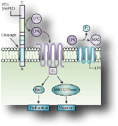 Autotaxin (ATX, ENPP2) is a secreted glycoprotein widely present in biological fluids, largely responsible for lysophosphatidic acid (LPA) production, a growth factor-like, signaling phospholipid. We have detected increased ATX and LPA levels, locally and/or systemically, in patients with different chronic inflammatory diseases and the corresponding animal models, including rheumatoid arthritis, hepatitis, as well as Idiopathic pulmonary fibrosis (IPF). With special regard to IPF, genetic or pharmacologic inhibition of ATX attenuated disease development in animal models, thus providing the proof of principle for therapeutic interventions and spurring three clinical trials. In this context, and using a combination of chemoinformatics and bioinformatics, rational design and targeted synthesis, dedicated enzymatic and ADMET assays, MS-based PK/PD analysis, as well as animal models and innovative delivery methods, we are developing a series of novel ATX inhibitors, currently at different pre-clinical characterization stages, protected by National and International patent applications in collaborations with the pharmaceutical industry.
Autotaxin (ATX, ENPP2) is a secreted glycoprotein widely present in biological fluids, largely responsible for lysophosphatidic acid (LPA) production, a growth factor-like, signaling phospholipid. We have detected increased ATX and LPA levels, locally and/or systemically, in patients with different chronic inflammatory diseases and the corresponding animal models, including rheumatoid arthritis, hepatitis, as well as Idiopathic pulmonary fibrosis (IPF). With special regard to IPF, genetic or pharmacologic inhibition of ATX attenuated disease development in animal models, thus providing the proof of principle for therapeutic interventions and spurring three clinical trials. In this context, and using a combination of chemoinformatics and bioinformatics, rational design and targeted synthesis, dedicated enzymatic and ADMET assays, MS-based PK/PD analysis, as well as animal models and innovative delivery methods, we are developing a series of novel ATX inhibitors, currently at different pre-clinical characterization stages, protected by National and International patent applications in collaborations with the pharmaceutical industry.
The lab is currently editing a special issue on "The Role of Autotaxin and LPA Signalling in Embryonic Development, Pathophysiology and Cancer" at IJMS.
Indicative selected publications
- Fotopoulou S, Oikonomou N, Grigorieva E, Nikitopoulou I, Paparountas T, Thanassopoulou A, Zhao Z, Xu Y, Kontoyiannis DL, Remboutsika E, Aidinis V 2010, "ATX expression and LPA signalling are vital for the development of the nervous system.", Dev Biol., Mar 15;339(2) , :451-64 [PubMed].
- Nikitopoulou I, Oikonomou N, Karouzakis E, Sevastou I, Nikolaidou-Katsaridou N, Zhao Z, Mersinias V, Armaka M, Xu Y, Masu M, Mills GB, Gay S, Kollias G, Aidinis V. 2012, "Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis.", J Exp Med., 2012 May 7; 209(5):925-33. Epub 2012 Apr 9. [PubMed].
- Oikonomou N, Mouratis MA, Tzouvelekis A, Kaffe E, Valavanis C, Vilaras G, Karameris A, Prestwich GD, Bouros D, Aidinis V. 2012, "Pulmonary Autotaxin Expression Contributes to the Pathogenesis of Pulmonary Fibrosis.", Am J Respir Cell Mol Biol, 2012 Jun 28 [PubMed].
- Kaffe E, Katsifa A, Xylourgidis N, Ninou I, Zannikou M, Harokopos V, Foka P, Dimitriadis A, Evangelou K, Moulas AN, Georgopoulou U, Gorgoulis VG, Dalekos GN, Aidinis V. 2016, "Hepatocyte Autotaxin expression promotes liver fibrosis and cancer", Hepatology, 2016 Dec 16. [PubMed].
- Magkrioti C, Oikonomou N, Kaffe E, Mouratis MA, Xylourgidis N, Barbayianni I, Megadoukas P, Harokopos V, Valavanis C, Chun J, Kosma A, Stathopoulos GT, Bouros E, Bouros D, Syrigos KN, Aidinis V. 2018, "The autotaxin - lysophosphatidic acid axis promotes lung carcinogenesis", Cancer Res. , 2018 May 3. [PubMed].
- Matralis AN, Afantitis A, Aidinis V. 2019, "Development and therapeutic potential of autotaxin small molecule inhibitors: From bench to advanced clinical trials", Med Res Rev., 2019 May; 39(3):976-1013 [PubMed].
- Magkrioti C, Galaris A, Kanellopoulou P, Stylianaki EA, Kaffe E, Aidinis V. 2019, "Autotaxin and chronic inflammatory diseases", J Autoimmun., 2019 Aug 27: 102327 [PubMed].
Older interests