Understanding the contribution of fibroblast heterogeneity and functions in intestinal damage and repair.
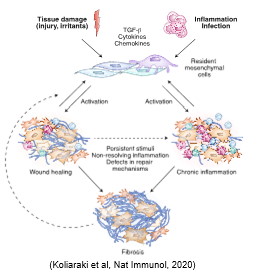 Tissue damage and inflammation in the intestine is followed by a series of events that result in mucosal repair, re-epithelization, and regeneration to restore normal organ function. These involve both intrinsic epithelial responses and microenvironmental alterations that are tightly regulated. Failure in any of these processes can lead to serious implications, including chronic inflammatory disorders and fibrosis. Despite the importance of intestinal fibroblasts in these processes, their contribution in terms of cellular and molecular mechanisms remains poorly understood. We believe that fibroblasts play an important role both in the orchestration of a regeneration-permissive microenvironmental milieu and the dictation of epithelial cell fate decisions. To address our hypothesis, our aim is to:
Tissue damage and inflammation in the intestine is followed by a series of events that result in mucosal repair, re-epithelization, and regeneration to restore normal organ function. These involve both intrinsic epithelial responses and microenvironmental alterations that are tightly regulated. Failure in any of these processes can lead to serious implications, including chronic inflammatory disorders and fibrosis. Despite the importance of intestinal fibroblasts in these processes, their contribution in terms of cellular and molecular mechanisms remains poorly understood. We believe that fibroblasts play an important role both in the orchestration of a regeneration-permissive microenvironmental milieu and the dictation of epithelial cell fate decisions. To address our hypothesis, our aim is to:
1) Characterize the heterogeneity and the cellular and molecular alterations of the damaged and regenerating intestinal stroma.
2) Delineate the cellular identities and fates of fibroblasts during intestinal damage and repair.
3) Elucidate the molecular mechanisms underlying fibroblasts’ functions in intestinal regeneration.
Selected publications
Melissari MT, Henriques A, Tzaferis C, Prados A, Sarris ME, Chalkidi N, Mavroeidi D, Chouvardas P, Grammenoudi S, Kollias G, Koliaraki V. (2021) Col6a1+/CD201+ mesenchymal cells regulate intestinal morphogenesis and homeostasis, Cell Mol Life Sci, 79(1):1.
Koliaraki V*, Prados A, Armaka M, Kollias G. (2020) The mesenchymal context in inflammation, immunity and cancer, Nat Immunol., 21(9):974-982.
The identification of CAF-specific cellular and molecular mechanisms that regulate tumor initiation and progression.
 The crucial role of the tumor microenvironment in the initiation and progression of cancer and specifically the importance of cancer-associated fibroblasts (CAFs) is now well established. However, the molecular mechanisms underlying their functions and their interactions with nearby cells, as well as the significance and specialized functions of distinct CAF subpopulations are still incompletely understood. Our aim is:
The crucial role of the tumor microenvironment in the initiation and progression of cancer and specifically the importance of cancer-associated fibroblasts (CAFs) is now well established. However, the molecular mechanisms underlying their functions and their interactions with nearby cells, as well as the significance and specialized functions of distinct CAF subpopulations are still incompletely understood. Our aim is:
1) To describe the heterogeneity of the tumor microenvironment in mouse models of intestinal carcinogenesis and analyze the activation trajectories of cancer-associated fibroblasts to infer their origin.
2) To explore the role of Notch3 as a regulator of cancer-associated fibroblast reprogramming and as a potential central node in a communication network between different cell types inside intestinal tumors, which should be location- and contact-specific.
3) To elucidate the role of fibroblast-derived IGF1 in bladder cancer, dissect its cell-specific mechanism of action and explore its therapeutic utility.
Our aim is to discover novel cellular and molecular mechanisms that play an important role in CAF reprogramming and function, to be able to utilize them in diagnosis and therapy.
Selected publications
Koliaraki V*, Prados A, Armaka M, Kollias G. (2020) The mesenchymal context in inflammation, immunity and cancer, Nat Immunol., 21(9):974-982.
Melissari MT, Chaklidi N, Sarris ME, Koliaraki V. (2020) Fibroblast reprogramming in gastrointestinal cancer, Front. Cell Dev. Biol., 8: 630-639.
Koliaraki V*, Chalkidi N, Henriques A, Tzaferis C, Polykratis A, Waisman A, Muller W, Hackam DJ, Pasparakis M, Kollias G. (2019) Innate sensing through mesenchymal TLR4/ MyD88 signals promote spontaneous intestinal tumorigenesis, Cell Rep, 26(3):536-546.e4.
Henriques A, Koliaraki V*, Kollias G. (2018) Mesenchymal MAPKAPK2/HSP27 drives intestinal carcinogenesis, Proc Natl Acad Sci USA. 115(24): E5546-E5555.
Koliaraki V, Pasparakis M, Kollias G. (2015) IKKβ in intestinal mesenchymal cells promotes initiation of colitis-associated cancer. J Exp Med. 212(13):2235-51.