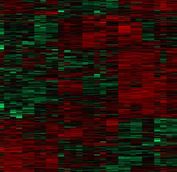
 1) The objective of our work is the definition of specific molecular events that induce Tau to become neurotoxic. This is mainly a proteomic project, based on the availability at Fleming of a state-of-the art proteomic facility. We are trying to establish successful strategies for the sensitive and deep proteomic identification of Tau interactome not only in the whole CNS of Drosophila but also in specific neuronal tissues (i.e only in the Mushroom Bodies, the Drosophila functional equivalent of the hippocampus). Finally, we are trying to develop fast and inexpensive protocols to allow the use of stable isotope dimethyl labelling for quantitative proteomics in line with the latest technologies and computational tools.
1) The objective of our work is the definition of specific molecular events that induce Tau to become neurotoxic. This is mainly a proteomic project, based on the availability at Fleming of a state-of-the art proteomic facility. We are trying to establish successful strategies for the sensitive and deep proteomic identification of Tau interactome not only in the whole CNS of Drosophila but also in specific neuronal tissues (i.e only in the Mushroom Bodies, the Drosophila functional equivalent of the hippocampus). Finally, we are trying to develop fast and inexpensive protocols to allow the use of stable isotope dimethyl labelling for quantitative proteomics in line with the latest technologies and computational tools.
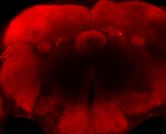
 2) In AD and all primary Tauopathies, Tau becomes increasingly phosphorylated with disease progression and self-assembles into neurofibrillary tangles (NFTs). The process of Tau aggregation, from oligomers to Neurofibrillary Tangles is suspected to induce dysfunction and neurodegeneration in AD and related Tauopathies. The field has been trying to define which specific aberrant Tau structure is toxic to neurons and to differentiate between the toxic effects that early Tau oligomers and later forms of fibrillar Tau exert to the cell. Other key questions such as the mechanism that initiates the aggregation of a highly soluble protein such as Tau in vivo also remain largely unanswered. Our work aims to address these questions by studying the interaction between Tau and proteins identified in our proteomic screen that could profoundly affect its aggregation propensity.
2) In AD and all primary Tauopathies, Tau becomes increasingly phosphorylated with disease progression and self-assembles into neurofibrillary tangles (NFTs). The process of Tau aggregation, from oligomers to Neurofibrillary Tangles is suspected to induce dysfunction and neurodegeneration in AD and related Tauopathies. The field has been trying to define which specific aberrant Tau structure is toxic to neurons and to differentiate between the toxic effects that early Tau oligomers and later forms of fibrillar Tau exert to the cell. Other key questions such as the mechanism that initiates the aggregation of a highly soluble protein such as Tau in vivo also remain largely unanswered. Our work aims to address these questions by studying the interaction between Tau and proteins identified in our proteomic screen that could profoundly affect its aggregation propensity.
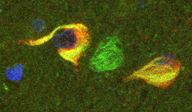
 3) In addition to its role as a microtubule-associated protein, Tau partners with the actin cytoskeleton and actin dynamics appear significantly altered in Tauopathies. The finding that Hirano bodies, eosinophilic inclusions found in the brains of patients with Alzheimer’s disease, are primarily composed of filamentous actin (F-actin) provided the first hint of an association between actin dysfunction and neurodegeneration. It has also been reported that destabilizing actin filaments in Drosophila, prevented Tau-induced degeneration, thus implicating alterations in actin dynamics as a mediator of Tau toxicity. We focus on proteins that are implicated in the regulation of actin dynamics and adopted that as selection criterion for the genetic validation of our proteomic results.
3) In addition to its role as a microtubule-associated protein, Tau partners with the actin cytoskeleton and actin dynamics appear significantly altered in Tauopathies. The finding that Hirano bodies, eosinophilic inclusions found in the brains of patients with Alzheimer’s disease, are primarily composed of filamentous actin (F-actin) provided the first hint of an association between actin dysfunction and neurodegeneration. It has also been reported that destabilizing actin filaments in Drosophila, prevented Tau-induced degeneration, thus implicating alterations in actin dynamics as a mediator of Tau toxicity. We focus on proteins that are implicated in the regulation of actin dynamics and adopted that as selection criterion for the genetic validation of our proteomic results.