Elucidate the role of adhesion in tumour angiogenesis.
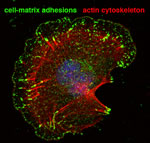 Cancer development and metastasis depends on efficient blood supply provided by co-option and expansion of the host vascular network. Indeed, angiogenesis is one of the 10 hallmarks of cancer. Strategies aiming to disrupt aberrant tumour angiogenesis and/or normalise the vasculature for efficient drug delivery, are extensively explored for anti-cancer therapies. Very little is known about how cell adhesion regulates tumour angiogenesis. Previous studies have shown that integrins, the main cell-matrix adhesion receptor could have both pro- and anti-angiogenic roles. Integrin activity depends on the formation and function of a dynamic network of signaling and cytoskeletal proteins localized at cell-matrix adhesion sites, named the integrin-adhesome. The proteins of the adhesome not only facilitate the physical anchorage of the cell to ECM and the regulation of adhesion strength necessary for cell migration and invasion, but also mediate the cross-talk of integrins with growth factors such as VEGF and bFGF, and the activation of intracellular signaling pathways leading to cell survival and proliferation. For this reason intensive efforts are directed over the recent years in elucidating the molecular mechanisms controlling the assembly and function of this adhesion network.
Cancer development and metastasis depends on efficient blood supply provided by co-option and expansion of the host vascular network. Indeed, angiogenesis is one of the 10 hallmarks of cancer. Strategies aiming to disrupt aberrant tumour angiogenesis and/or normalise the vasculature for efficient drug delivery, are extensively explored for anti-cancer therapies. Very little is known about how cell adhesion regulates tumour angiogenesis. Previous studies have shown that integrins, the main cell-matrix adhesion receptor could have both pro- and anti-angiogenic roles. Integrin activity depends on the formation and function of a dynamic network of signaling and cytoskeletal proteins localized at cell-matrix adhesion sites, named the integrin-adhesome. The proteins of the adhesome not only facilitate the physical anchorage of the cell to ECM and the regulation of adhesion strength necessary for cell migration and invasion, but also mediate the cross-talk of integrins with growth factors such as VEGF and bFGF, and the activation of intracellular signaling pathways leading to cell survival and proliferation. For this reason intensive efforts are directed over the recent years in elucidating the molecular mechanisms controlling the assembly and function of this adhesion network.
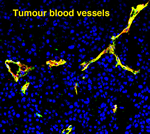 Recently, we examined the function of Focal adhesion kinase (FAK), a crucial adhesion regulator in tumour angiogenesis. FAK integrates signals from growth factor receptors and integrins to co-ordinate a large variety of cellular responses. In close collaboration with Dr Hodivala-Dilke’s lab (The Barts Institute, CR-UK), we established that FAK controls pathological angiogenesis. In particular, we demonstrated that partial reduction, in contrast to complete elimination of FAK, affects endothelial cell function, by increasing cell proliferation and sprout formation. These studies indicate a dose-dependent effect of FAK in pathological angiogenesis, complementing on-going clinical trials with FAK inhibitors. Current projects in the lab investigate additional key regulators of adhesion dynamics, including Talin, ILK and PINCH, in tumour angiogenesis.
Recently, we examined the function of Focal adhesion kinase (FAK), a crucial adhesion regulator in tumour angiogenesis. FAK integrates signals from growth factor receptors and integrins to co-ordinate a large variety of cellular responses. In close collaboration with Dr Hodivala-Dilke’s lab (The Barts Institute, CR-UK), we established that FAK controls pathological angiogenesis. In particular, we demonstrated that partial reduction, in contrast to complete elimination of FAK, affects endothelial cell function, by increasing cell proliferation and sprout formation. These studies indicate a dose-dependent effect of FAK in pathological angiogenesis, complementing on-going clinical trials with FAK inhibitors. Current projects in the lab investigate additional key regulators of adhesion dynamics, including Talin, ILK and PINCH, in tumour angiogenesis.
Understanding how endothelial cell-matrix adhesions control vascular morphogenesis could provide an alternative effective strategy for blood vessel intervening therapies.
Indicative selected publications:
Kostourou V* , Lechertier T, Reynolds LE, Lees DM, Baker M, Jones DT, Tavora B, Ramjaun AR, Birdsey GM, Robinson SD, Parsons M, Randi AM, Hart IR, Hodivala-Dilke K. 2013, "FAK-heterozygous mice display enhanced tumour angiogenesis.", Nat Commun. , 2013; 4:2020. doi:10.1038/ncomms3020.
Press release from Nature Publishing Group office and highlight in Nature.com web page.
Tavora B, Batista S, Reynolds LE, Jadeja S, Robinson S, Kostourou V , Hart I, Fruttiger M, Parsons M, Hodivala-Dilke KM. 2010, "Endothelial FAK is required for tumour angiogenesis.", EMBO Mol Med. , 2010 Dec;, 2(12):516-28. [PubMed].
da Silva RG, Tavora B, Robinson SD, Reynolds LE, Szekeres C, Lamar J, Batista S, Kostourou V, Germain MA, Reynolds AR, Jones DT, Watson AR, Jones JL, Harris A, Hart IR, Iruela-Arispe ML, Dipersio CM, Kreidberg JA, Hodivala-Dilke KM. 2010, "Endothelial {alpha}3{beta}1-Integrin Represses Pathological Angiogenesis and Sustains Endothelial-VEGF.", Am J Pathol. , 2010 Sep;177(3):1534-48. [PubMed].
Germain M, De Arcangelis A, Robinson SD, Baker M, Tavora B, D'Amico G, Silva R, Kostourou V, Reynolds LE, Watson A, Jones JL, Georges-Labouesse E, Hodivala-Dilke K. 2010, "Genetic ablation of the alpha 6-integrin subunit in Tie1Cre mice enhances tumour angiogenesis", J Pathol., 2010 Feb;220(3):370-81. [PubMed].
Robinson SD, Reynolds LE, Kostourou V, Reynolds AR, da Silva RG, Tavora B, Baker M, Marshall JF, Hodivala-Dilke KM. 2009, "Alphav beta3 integrin limits the contribution of neuropilin-1 to vascular endothelial growth factor-induced angiogenesis", J Biol Chem. 2, 2009 Dec 4; 84(49):33966-81. [PubMed].
Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V400. [PubMed].
Claxton S, Kostourou V, Jadeja S, Chambon P, Hodivala-Dilke K, Fruttiger M. 2008, "Efficient, inducible Cre-recombinase activation in vascular endothelium.", Genesis. , 2008 Feb; 46(2):74-80. [PubMed].
Delineate the function of adhesion in developmental angiogenesis
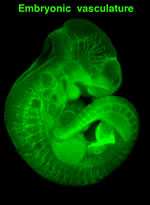 The vascular system is the first system to form during embryogenesis, indicating its vital role for organism development and survival. The initial vascular plexus is generated by vasculogenesis, the process of de novo synthesis of blood vessels from angioblasts and endothelial progenitor cells. Subsequent remodeling of the vascular tree occurs via angiogenesis and involves the expansion of the vascular network by circumferential growth, sprouting and splitting. During angiogenesis, re-organisation of endothelial cell adhesions is a prerequisite for various processes necessary for new blood vessel assembly, including extracellular matrix remodelling, lumen formation, proliferation and invasion. Using functional genetic approaches, in collaboration with David Critchley’s lab (University of Leicester, UK) we demonstrated that Talin, a key regulator and determinant of integrin function, plays a crucial role in embryonic angiogenesis.
The vascular system is the first system to form during embryogenesis, indicating its vital role for organism development and survival. The initial vascular plexus is generated by vasculogenesis, the process of de novo synthesis of blood vessels from angioblasts and endothelial progenitor cells. Subsequent remodeling of the vascular tree occurs via angiogenesis and involves the expansion of the vascular network by circumferential growth, sprouting and splitting. During angiogenesis, re-organisation of endothelial cell adhesions is a prerequisite for various processes necessary for new blood vessel assembly, including extracellular matrix remodelling, lumen formation, proliferation and invasion. Using functional genetic approaches, in collaboration with David Critchley’s lab (University of Leicester, UK) we demonstrated that Talin, a key regulator and determinant of integrin function, plays a crucial role in embryonic angiogenesis. 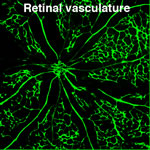 Specific deletion of Talin in endothelial cells using the Tie2-Cre caused embryonic lethality with severe hemorrhaging and vascular defects. On-going projects address the role of Talin and other adhesion regulators in later stages of developmental vascular patterning and in adult physiological angiogenesis during wound healing. Additionally, we employ the retina neovascularization model, to further characterize and identify specific angiogenic processes where adhesion proteins play a fundamental role. Targeting selected angiogenic steps could have an advantage over existing global anti-vascular strategies, because of minimizing deleterious effects in the established vasculature.
Specific deletion of Talin in endothelial cells using the Tie2-Cre caused embryonic lethality with severe hemorrhaging and vascular defects. On-going projects address the role of Talin and other adhesion regulators in later stages of developmental vascular patterning and in adult physiological angiogenesis during wound healing. Additionally, we employ the retina neovascularization model, to further characterize and identify specific angiogenic processes where adhesion proteins play a fundamental role. Targeting selected angiogenic steps could have an advantage over existing global anti-vascular strategies, because of minimizing deleterious effects in the established vasculature.
Indicative selected publications:
Monkley SJ, Kostourou V , Spence L, Petrich B, Coleman S, Ginsberg MH, Pritchard CA, Critchley DR. 2010, "Endothelial cell talin1 is essential for embryonic angiogenesis.", Dev Biol. , 2011 Jan 15; 349(2):494-502. [PubMed].
D'Amico G, Jones DT, Nye E, Sapienza K, Ramjuan AR, Reynolds LE, Robinson SD, Kostourou V, Martinez D, Aubyn D, Grose R, Thomas GJ, Spencer-Dene B, Zicha D, Davies D, Tybulewicz V, Hodivala-Dilke KM. 2009, "Regulation of lymphatic-blood vessel separation by endothelial Rac1.", Development. , 2009 Dec;136(23):4043-53. Erratum in: Development. 2010 Jan;137(2):359. [PubMed].
Defining endothelial adhesion properties in healthy and pathological conditions using global-omics approaches
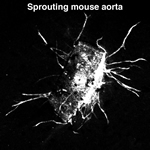 Endothelial cells respond to a variety of chemical and mechanical cues produced in their local microenvironment and become activated during angiogenesis and in vascular pathologies. We undertake large-scale proteomics and genomics studies to characterise the molecular composition of adhesion sites in healthy-quiescent and diseased-activated endothelial cells. Additionally, using our genetic mouse models and global –omics approaches in collaboration with Fleming proteomic and genomic facilities, we explore the molecular and cellular signature of specific alterations in endothelial cell adhesion properties.
Endothelial cells respond to a variety of chemical and mechanical cues produced in their local microenvironment and become activated during angiogenesis and in vascular pathologies. We undertake large-scale proteomics and genomics studies to characterise the molecular composition of adhesion sites in healthy-quiescent and diseased-activated endothelial cells. Additionally, using our genetic mouse models and global –omics approaches in collaboration with Fleming proteomic and genomic facilities, we explore the molecular and cellular signature of specific alterations in endothelial cell adhesion properties. 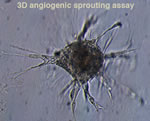 To increase our analysis tools and facilitate the validation of identified candidates we are optimising and developing angiogenic and molecular assays, using 4D-live imaging approaches in close collaboration with Fleming imaging facility. These studies are anticipated to reveal novel targets for regulating adhesion dynamics and endothelial cell activation, leading to the discovery of effective treatments for several vascular pathologies and cancer.
To increase our analysis tools and facilitate the validation of identified candidates we are optimising and developing angiogenic and molecular assays, using 4D-live imaging approaches in close collaboration with Fleming imaging facility. These studies are anticipated to reveal novel targets for regulating adhesion dynamics and endothelial cell activation, leading to the discovery of effective treatments for several vascular pathologies and cancer.
Indicative selected publications
Ikonomou G, Kostourou V , Shirasawa S, Sasazuki T, Samiotaki M, Panayotou G. 2012, "Interplay between oncogenic K-Ras and wild-type H-Ras in Caco2 cell transformation.", J Proteomics., 2012 Sep 18; 75(17):5356-69. doi: 10.1016/j.jprot.2012.06.038. [PubMed].