ERC Advanced grant awarded to George Kollias, Fleming Associated Researcher and Professor, Director of Physiology at the Medical School of Athens. The 2.4-million-euro project is for 5 years and will be hosted at BSRC Fleming.
The European Research Council (ERC) has awarded a prestigious ERC Advanced grant to Prof. George Kollias. George Kollias is Associated Researcher at BSRC Fleming and Professor and Director of the Department of Physiology at the Medical School of the National and Kapodistrian University of Athens. His lab focuses on understanding molecular and cellular mechanisms orchestrating complex phenotypes in chronic inflammation, immunity and cancer.
George Kollias will now receive 2.4 million euro for five years to implement his project BecomingCausal, titled “Contextual specification of fibroblast-driven causalities in chronic intestinal inflammation and fibrosis”. This is the second ERC grant for Prof. Kollias, following the highly successful Advanced ERC project MCs-inTEST (2014-2019).
Official ERC Press release: https://erc.europa.eu/news/erc-2021-advanced-grants-results
ERC AdG 2021 in figures | Indicative statistics: erc-2021-adg-statistics
ERC AdG 2021 results – All domains: erc-2021-adg-results-all-domains
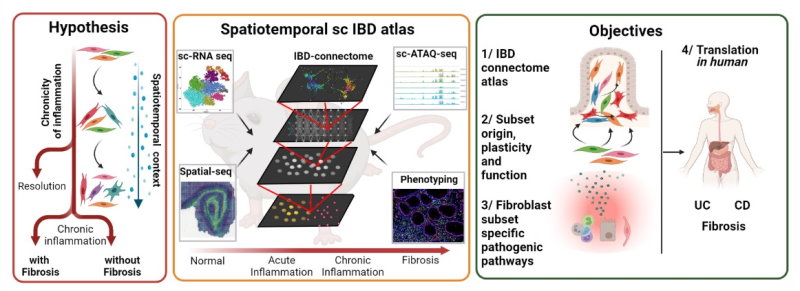
Project Abstract - Inflammatory bowel disease (IBD) is a severe, chronic pathology presenting with progressive intestinal inflammation and fibrosis, whose exact causes and key pathways remain poorly defined. Stromal – immune cell interactions have recently gained momentum in conceptualizing tissue homeostasis and our lab offered solid evidence establishing fibroblast heterogeneity and dominant roles in intestinal pathophysiology. Our recent preliminary evidence, indicated diverse spatial distribution of subsets of activated fibroblasts and revealed synergistic interplays of important inflammatory pathways driving pathogenicity. Detailed insights into such contextual complexities remain obscure. Here, we propose a novel unifying hypothesis that progressive IBD is orchestrated by specific subsets of fibroblasts, becoming causal to pathogenesis, depending on contextual information dictated by origin, topology and cross-talks with immune or stromal cell types.
We propose to use single-cell spatiotemporal phenotyping to deconvolute fibroblast subset-specific functions in disease-staged, fibrotic and non-fibrotic animal models of IBD. We aim to: (1) Map dynamic chromatin and gene expression programs that define cellular heterogeneity and infer cell interactions to build an ‘IBD connectome’ atlas (2) Analyse the origin, spatial distribution, plasticity and lineage trajectories of intestinal fibroblasts and reveal potential functions of pathogenic subsets (3) Perform discovery screens and functional validations on known (TNF, IFNγ, TGFb and interleukins) and novel fibroblast-subset-specific pathways focusing on potential synergistic interplays (4) Employ clinical material to validate involvement of the most prominent new pathways in human.
The proposed research should aid tackle the complexities of chronic inflammatory and fibrotic disorders in the intestine and beyond, help advance mechanistic concepts in immune disease pathophysiology and promote fibroblast-targeting therapeutic discovery.