19/12/2016
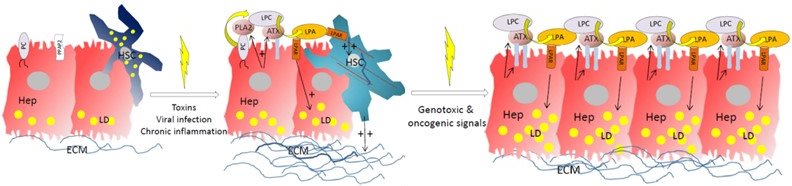
Liver injury in both mice and humans stimulates liver phospholipase A2 (PLA2) expression and the generation of lysophosphatyidylcholine (LPC) from membrane posphatiodylcholine (PC) moieties. LPC, in concert with lever inflammation and/or viral infection, then stimulate hepatocyte-specific Autotaxin (ATX) expression resulting to LPC hydrolysis and local lysophosphatidic acid (LPA) production, whose levels are further sustained by the dowregulation of lipid phosphatase PPA2AB, its main catabolizing enzyme extracellularly. In turn, the increased levels of LPA activate hepatic stellate cells (HSCs) and pro-fbrotic gene expression, while shifting the angiocrine response of liver sinusoidal endothelial cells and enforcing a pro-fibrotic vascular niche. Conditional genetic deletion or transgenic overexpression of ATX from hepatocytes modulated accordingly lipid homeostasis and liver pathophysiology, while pharmacologic ATX inhibition abrogated the development of fibrosis, thus suggesting ATX as a possible therapeutic target in chronic liver diseases (CLDs). Moreover, the pro-fibrotic effects of ATX were shown to create, in the presence of genotoxic signals, a more permissive microenvironment for the development of hepatocellular carcinoma (HCC), well consistent with reported LPA and GPCR properties. In addition, the ATX/LPA axis through the induction of stearoyl-CoA desaturase 2 (Scd2) expression and possibly other lipogenic genes, stimulates de novo fatty acid synthesis and triglyceride accumulation, further nurturing malignant growth.