New study from Fleming researchers published in Nature Immunology highlights fibroblastic mesenchymal cells as orchestrators of immunity
A new study published in Nature Immunology by the Kollias and Koliaraki labs in collaboration with the Ludewig lab at the University of Zurich, examines the development of fibroblastic reticular cells (FRCs) during lymphoid organogenesis in gut Peyer’s patches (PPs). The study shows that FRCs develop from two separate mesenchymal cell lineages, which form mosaic microenvironments and converge to support development of Follicular Dendritic Cells (FDCs) and antibody producing germinal centres (GCs) promoting immune cell activation and maintaining intestinal homeostasis.
https://doi.org/10.1038/s41590-021-00894-5
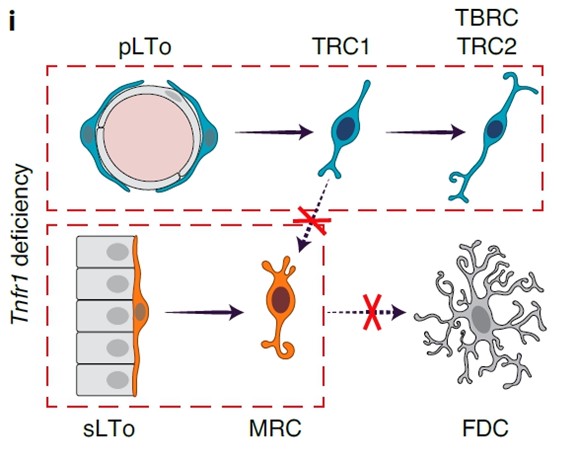
Secondary lymphoid organ development depends on the interaction of hematopoietic lymphoid tissue inducer (LTi) cells with resident mesenchymal and endothelial lymphoid tissue organizer (LTo) cells. PP organogenesis is initiated at embryonic day (E) 16.5, when LTi cells form clusters along the intestinal wall and induce the differentiation of LTo cells. This process requires lymphotoxin-β receptor (LTβR)-dependent activation of mesenchymal LTo cells. During the first week of life, T and B lymphocytes seed the PP primordium, accumulate in proximity to the muscular layerand drive the development of distinct FRC niches.
The study by Kollias, Koliaraki and Ludewig labs shows that PP FRCs develop from two separate mesenchymal LTo cell lineages. Perivascular and subepithelial PP FRC lineages converge and form mosaic microenvironments in an LTβR- and tumor necrosis factor receptor (TNFR) 1-dependent manner. The cooperation of the two FRC lineages in PPs is crucial for the maintenance of intestinal homeostasis and the induction of IgA responses, including those important for defense against mouse coronavirus.
FRC convergence from different fibroblastic progenitor populations and the utilization of complementary mechanisms provides a fail-safe mechanism for the generation of immune-stimulating environments, not only in secondary lymphoid organs, but also in differentiated inflammatory lesions that are commonly known as tertiary lymphoid structures. In the future, it will be interesting to investigate whether the existence of different fibroblast sources and the mechanisms that have been uncovered can also contribute to development of other tissues and participate in pathologies, such as chronic inflammation, fibrosis or tumorigenesis.