12/01/2015
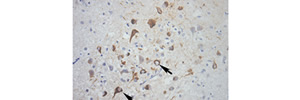
Excessive phosphorylation on the Neuronal protein Tau has been linked to neurodegenerative dementias including Alzheimer’s Disease. However, it has been unclear whether particular of the at least 85 potential phosphorylations are necessary and sufficient for the dementia-linked neuronal dysfunction and neuronal death underlying degeneration. Using Drosophila and mouse models as well as patient samples, Papanikolopoulou & Skoulakis demonstrate that Tau-mediated neuronal dysfunction and toxicity are separable and in fact neuronal dysfunction, which requires phosphorylation on Ser262, precedes and is required for Ser238 phosphorylation which in turn is requisite for premature lethality. These results coupled with novel antibodies targeting phospho Ser238 which constitute excellent predictive Tauopathy biomarkers, also suggest these phosphorylation sites as promising targets for pathology ameliorating interventions.
In the image, the anti-phospho Ser238 antibody stains pathological neurons (Brown) in the the hippocampus of a an Alzheimer’s patient.