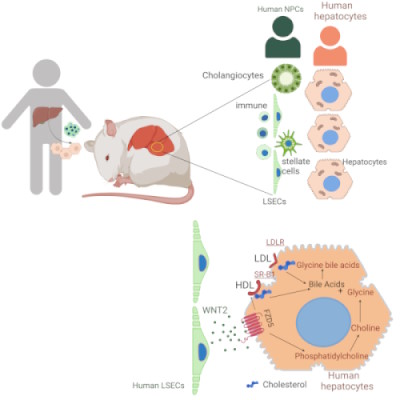
Humanized mouse liver reveals endothelial control of essential hepatic metabolic functions
A comprehensive human liver tissue was established in a mouse host that consists of all human-relevant parenchymal and non-parenchymal cell types and mimics the cellular composition, histological architecture, and functional properties of a human liver. This highly human-relevant murine model allows investigation of human-specific metabolic features and liver cell type interactions. Using this model it was found that essential hepatocyte functions like cholesterol uptake and bile acid conjugation are not cell autonomous but are highly dependent on signals from the adjacent endothelial cells. This paracrine regulation of essential hepatocyte specific metabolic functions is disrupted in the context of fibrosis due to collagen deposition between hepatocytes and endothelial cells. Dysfunctional hepatocytes are a major cause of mortality in liver disease patients. Knowing the signals that hepatocyte require from their environment to function properly may help to improve hepatocyte function at the stage of advanced fibrosis.
The project was led by E. Kaffe, a close collaborator of the lab, in the context of a granted Hellenic Research Foundation (ELIDEK) research grant.
[PubMed]